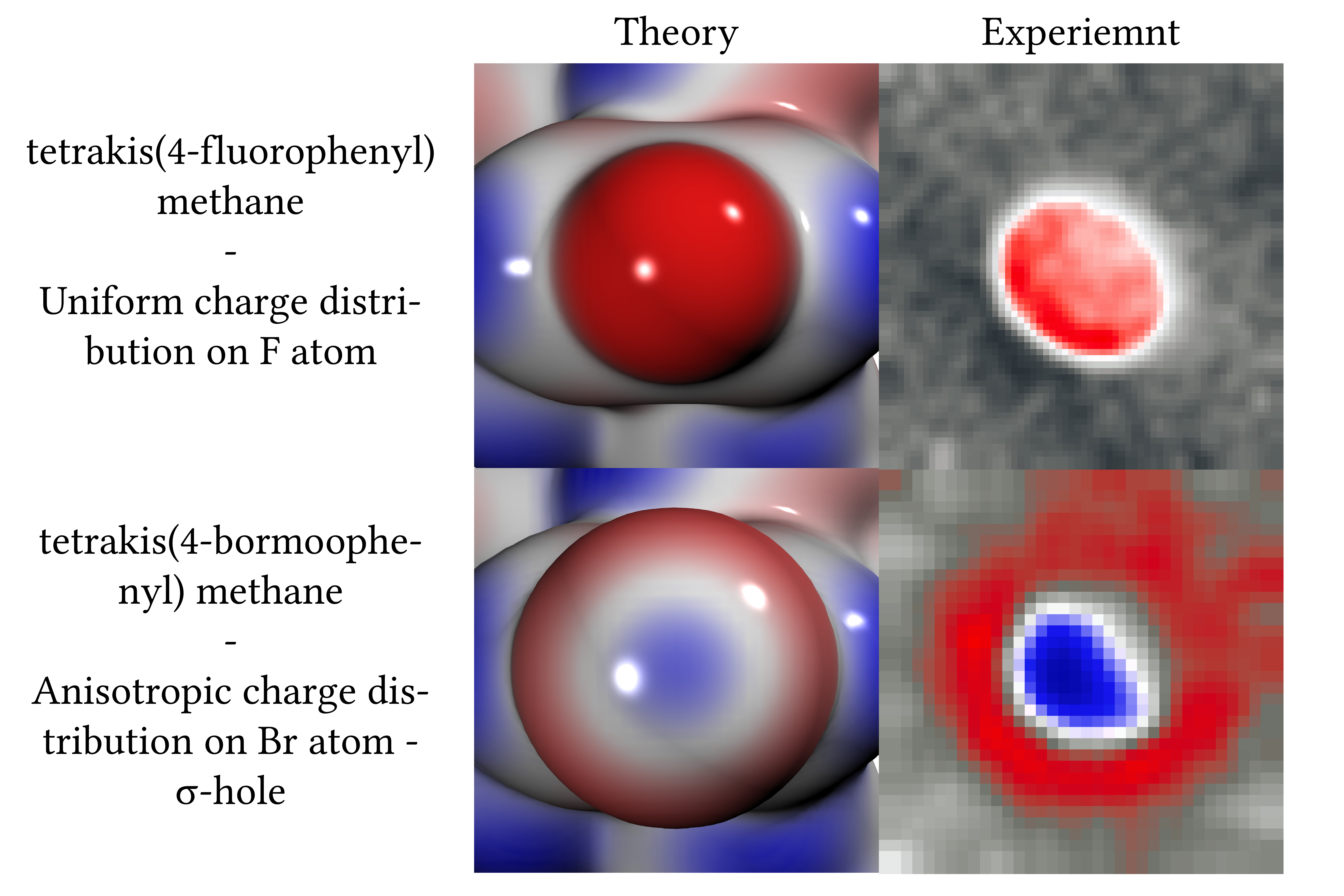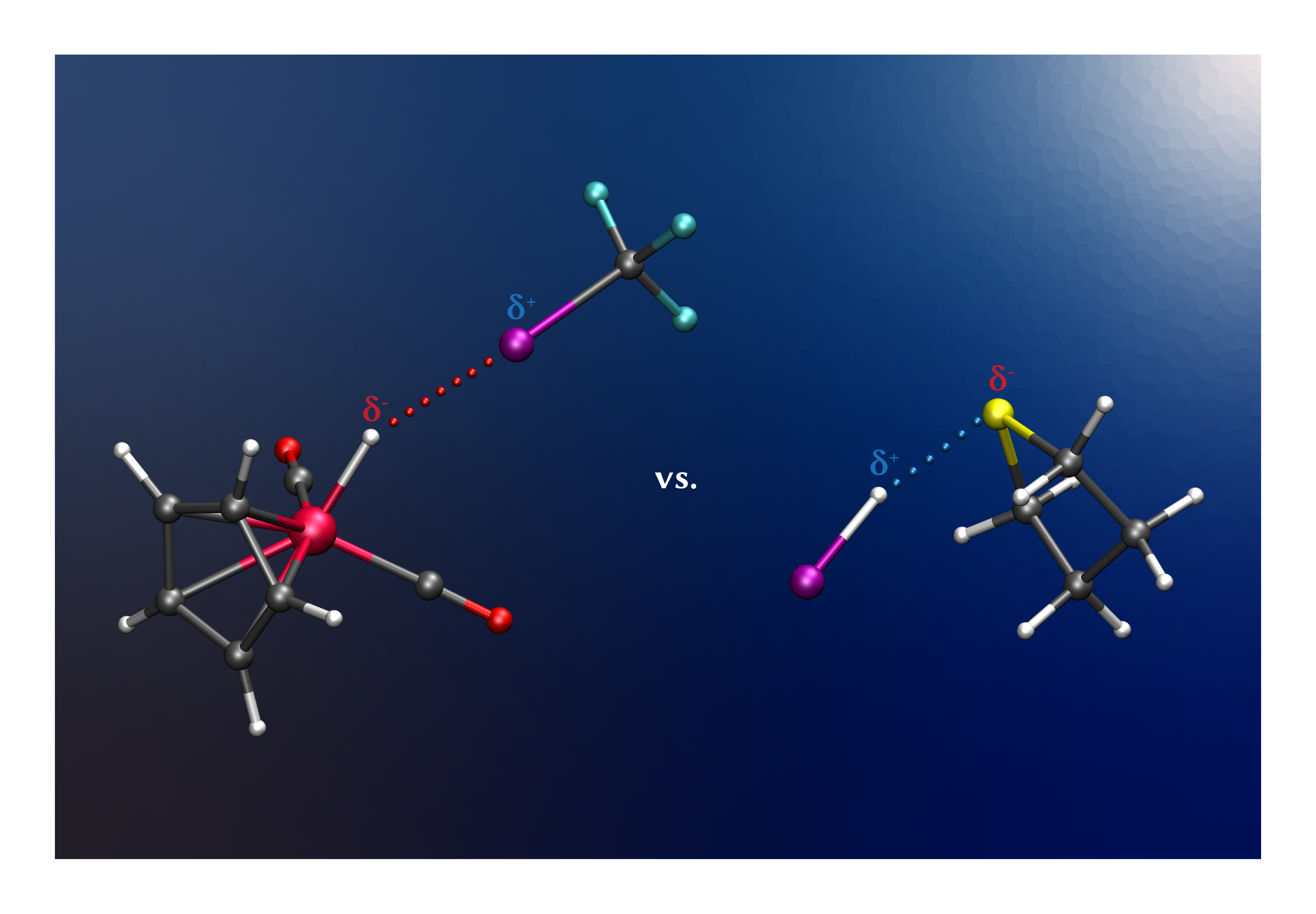
Hydrogen bonding: protonic & hydridic
Noncovalent interactions—especially hydrogen bonds with protonic or hydridic hydrogen—are probed using low-temperature IR spectroscopy combined with high-level electronic-structure theory. Similar spectral fingerprints are seen in both cases: the X–H stretch red-shifts and intensifies upon complexation, with comparable stabilization energies across main-group and transition-metal systems. Mechanistically, protonic H-bonding is dominated by electrostatics, whereas hydridic H-bonding is dominated by London dispersion from highly polarizable hydrides, supporting a unified view of hydrogen bonding and motivating a refined definition.